Blog
Key Insights on Improving Site Efficiency from Outsourcing in Clinical Trials East Coast

The 15th Annual Outsourcing in Clinical Trials East Coast conference provided a wealth of insights on how technology can aid in clinical trial management with presentations from industry leaders in life sciences and pharmaceuticals. This year’s event also spotlighted the pivotal role sponsors play in reducing the administrative burdens placed on sites. Read on for some key takeaways on these challenges and how sponsors can easily and effectively eliminate one of the major burdens imposed by technology.
Clinical Trial Site Technical and Operational Challenges
Imagine you’re a clinical research coordinator juggling multiple studies, each with several sites and their own virtual mountain of email chains, applications, and logins. For everyone involved, from investigators to site staff, managing all these disconnected systems and communication streams eats up valuable time better spent elsewhere.
When sites are bogged down with administrative tasks, it slows the entire research process. And it’s a major reason why some sites are walking away from sponsor contracts. The good news? There are simple solutions that make a big impact.
Suzy Montanye, the Site Relationship Manager at Endo Pharmaceuticals, delivered one of the most powerful talks at the conference on actionable ways to improve the trial process for sponsors and sites alike. In her talk, Site Management: Building a Strong Partnership with Sites to Ensure You Are a Sponsor of Choice, she emphasized that sponsors have an opportunity to relieve challenges sites face by addressing some key operational and financial burdens.
How to Become the Sponsor of Choice for Clinical Trial Sites
To stand out and become a preferred sponsor, it’s crucial to take strategic actions to support sites’ needs. Much of the time-consuming work sites undertake goes uncompensated, so investing in easing their operational burdens goes a long way in improving the interdependent site-sponsor relationship.
For example, challenges during the data collection process can hinder site operations. Inadequate training on the data entry systems provided by sponsors leads to errors in data collection and consumes valuable time. When these difficulties arise during the patient screening process, especially when staff must stop to troubleshoot technical issues, patient retention and trial completion are both at risk.
Sponsors have the power to alleviate these stresses and improve site performance through the following three strategies:
- Streamlining Access: Reduce the burden of siloed systems and numerous passwords by employing user-friendly technology solutions.
- Effective Communication: Save time by automating communications, such as administrative emails for user onboarding during the study start-up phase.
- Providing the Right Tools: Equip sites with the necessary technology and resources to efficiently perform tasks, such as data entry, user access, and technology training.
It’s a smart approach to first target high-impact, easy-to-implement areas for improvement. Notably, simplifying password management using a trusted single sign-on solution is a key area where sponsors can make an immediate difference that is well worth the initial investment.
Too Many Passwords: The #1 Technical Challenge for Clinical Trial Sites
Yes, it seems mundane. But password management remains a problem.
In a survey conducted by the Society for Clinical Research Sites (SCRS) in 2020, 74% of sites polled reported that “keeping track of multiple usernames and passwords (is) a top technology challenge.” That white paper, titled Sites Speak Out on Clinical Trial Technology Overload, went on to detail that sites experience a deluge of siloed applications, requiring staff to often enter information multiple times in separate systems, all with different credentials.
For instance, a typical study may involve close to 50 passwords. Standalone systems require staff to learn how to navigate distinct portals and variable password requirements. While keeping track of numerous passwords can disrupt workflow and efficiency, more significantly, they pose a risk if site staff resort to poor cybersecurity hygiene, such as writing down or sharing login information.
One Password, One Solution: How Single Sign-On Solves These Challenges
Sponsors have a golden opportunity to become more desirable industry partners by enabling one universal password for all applications—especially if the application is one a site already uses by virtue of their work with other sponsors. This provides not only a day-to-day convenience for users but also allows both administrators and users to skip a tedious application onboarding process where credentials must be issued and authorizations must be tracked and granted in lengthy, manual approval cycles. Without a universal password, those processes can delay critical start-up activities and exacerbate time pressures for sites.
Today, Exostar’s Secure Access Manager (SAM)™ provides the majority of large sponsors’ site users with one username and password across all sites, studies, and sponsors. Sponsors can allow users to apply their existing Exostar® credentials, rather than creating and maintaining new credentials for each application in their study. This saves major time and frustration, especially during the all-important start-up phase, empowering clinical trials to meet time-to-market objectives. Plus, better password security practices eliminate risks to valuable sensitive information and intellectual property.
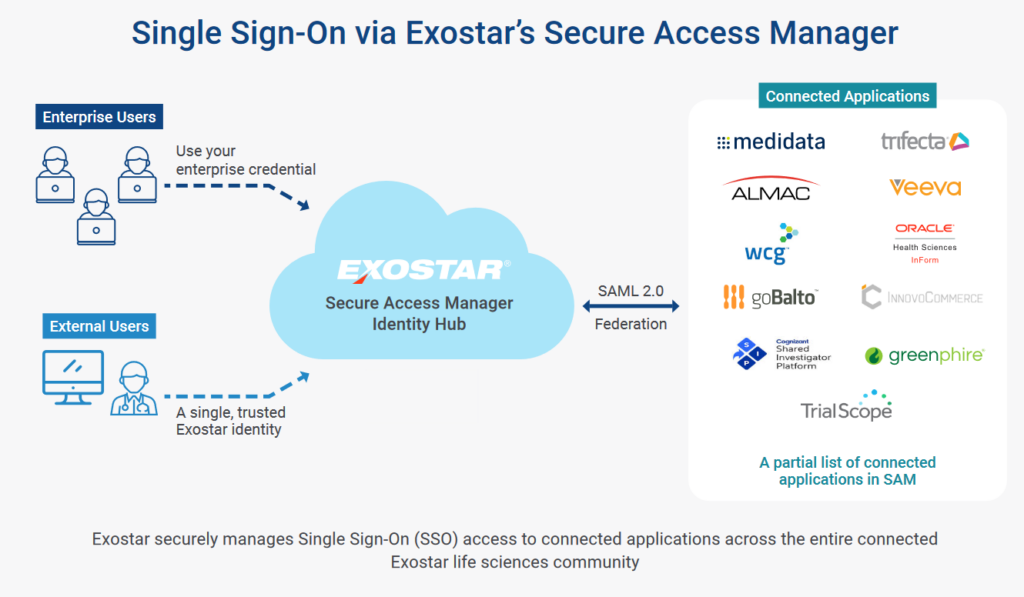
Because SAM™ also features a trusted community of life science members operating under established security safeguards, sponsors can accept these credentials with confidence, avoiding altogether the pitfalls of accepting social authentication.
Get Started Today
The Outsourcing in Clinical Trials East Coast conference highlighted the pressing need for sponsors to implement better technology solutions to streamline clinical trial management and reduce security risks. Luckily, these solutions are already out there.
Find out more about how Secure Access Manager™ can help you accelerate the trial process and become the partner of choice for clinical trial sites. 25 of the top global pharmaceutical companies already use SAM™ to improve their workplace efficiency and security. Please contact our solution experts today to get started and join our growing life sciences community.